- Menu
- Search For Product Models
Search For Product Models
- cn
In the food and pharmaceutical industries, hygiene, contamination control, and regulatory compliance are non-negotiable requirements. Heat exchangers used in these sectors must meet strict sanitary standards to ensure product safety, process integrity, and long-term reliability.
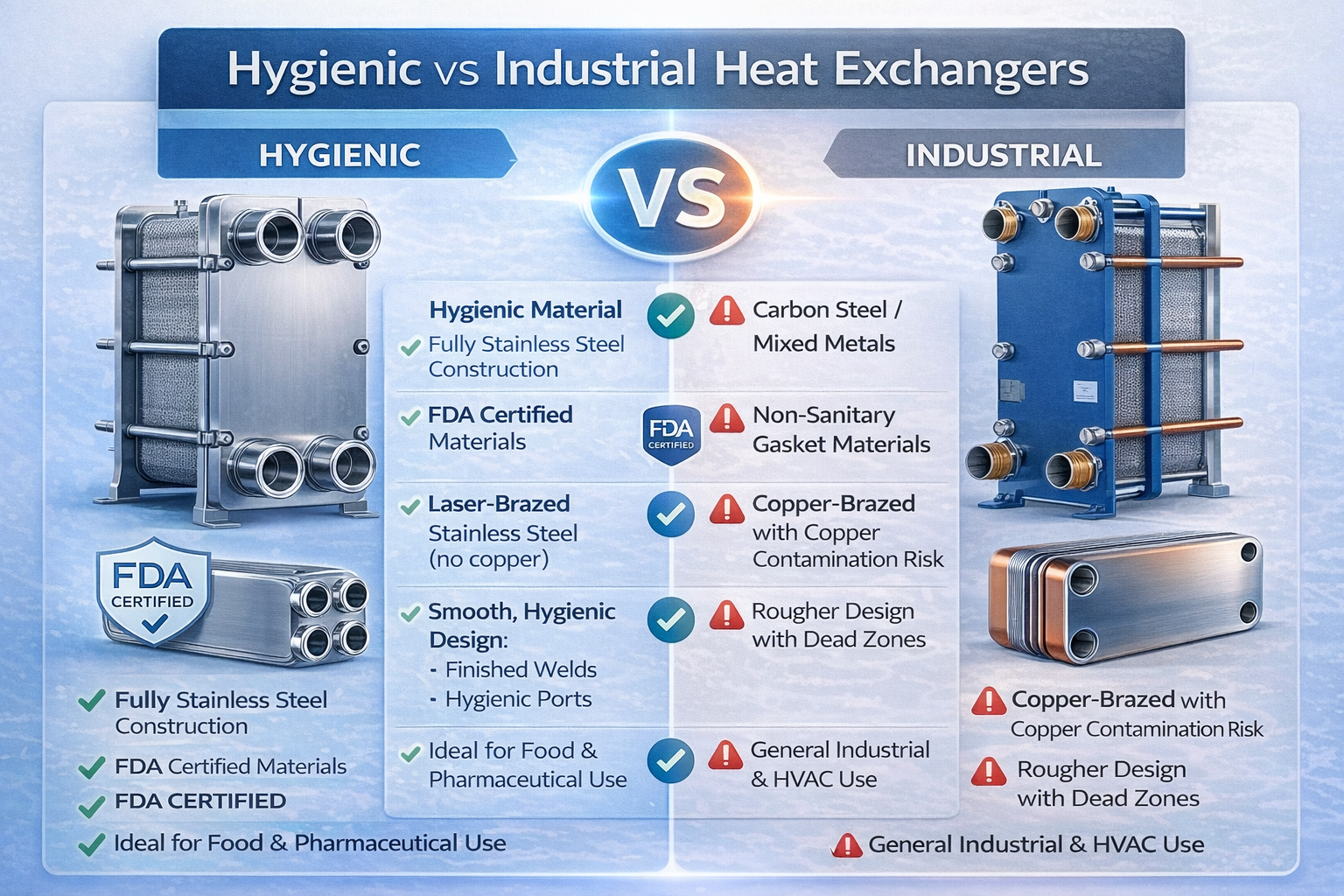
For this reason, fully stainless steel hygienic heat exchangers are the standard solution. For gasketed units, all wetted parts must be stainless steel with FDA-certified gaskets. For brazed units, only fully stainless steel fusion-bonded designs are acceptable.
Food and pharmaceutical processes involve direct contact with consumable or injectable products. Any contamination from materials can lead to:
Product recalls
Regulatory violations
Severe health risks
Brand damage
Copper, carbon steel, or mixed-metal components may release ions or corrode under cleaning conditions. Fully stainless steel construction eliminates the risk of metallic contamination.
Materials typically used:
AISI 316L (preferred)
AISI 304 (non-critical zones)
316L is preferred due to its superior corrosion resistance and low carbon content, which minimizes carbide precipitation during welding.
Food and pharmaceutical systems undergo regular:
CIP (Clean-in-Place)
SIP (Sterilize-in-Place)
These processes involve:
High temperature
Strong alkaline solutions
Acid cleaning agents
Steam sterilization
Materials must resist:
Chemical corrosion
Thermal cycling
Surface degradation
Fully stainless steel heat exchangers provide stable performance under repeated cleaning and sterilization cycles.
Sanitary heat exchangers must comply with:
Smooth surface finish (Ra ≤ 0.8 μm typical)
No dead zones
No crevices
Easy drainage
Fully welded hygienic ports
Mixed-material brazed heat exchangers (such as copper brazed) are not suitable because:
Copper is not accepted in pharmaceutical-grade systems
Brazing filler metals may create contamination risk
Internal inspection is impossible
For gasketed plate heat exchangers used in food or pharma:
All gaskets must be:
FDA compliant
EU 1935/2004 compliant (if exporting to Europe)
Suitable for food contact
Common materials:
EPDM (FDA grade)
NBR (food grade)
FKM (special applications)
Non-certified elastomers can:
Leach plasticizers
Absorb product
Degrade under CIP conditions
Create microbiological risks
Therefore, all wetted components must be stainless steel + FDA-certified gasket materials.
Traditional brazed plate heat exchangers use:
Copper brazing filler
Nickel brazing filler
These are not recommended for hygienic applications because:
Copper can contaminate product
Nickel alloys may not meet pharmaceutical standards
Brazed joints cannot be inspected internally
Fully stainless steel fusion-bonded heat exchangers (laser welded or vacuum welded) eliminate filler metals and provide:
Pure stainless steel wetted surface
High structural integrity
Compliance with hygienic standards
No cross-contamination risk
Food and pharmaceutical heat exchangers must comply with:
FDA (USA)
3-A Sanitary Standards
EHEDG (Europe)
GMP regulations
ASME BPE (pharmaceutical piping standard)
Using non-sanitary materials can result in:
Audit failure
Production shutdown
Certification loss
Hygienic heat exchangers must:
Drain completely
Avoid product entrapment
Withstand frequent disassembly (for gasketed types)
Maintain surface integrity after years of cleaning
Fully stainless steel construction ensures:
No galvanic corrosion
No material mismatch
Stable long-term operation
In food and pharmaceutical industries, material selection is not only about corrosion resistance — it is about compliance, safety, and liability control.
Therefore:
✔ Gasketed heat exchangers must be fully stainless steel with FDA-certified gaskets.
✔ Brazed units must be fully stainless steel fusion-bonded (no copper filler).
✔ Surface finish and hygienic design must meet sanitary standards.
Choosing the correct hygienic heat exchanger protects product quality, regulatory compliance, and brand reputation.
Copper-brazed heat exchangers may release trace metal ions under acidic or CIP conditions.
In food and pharmaceutical processes, any risk of metallic contamination is unacceptable. Additionally, brazed joints cannot be visually inspected internally, which conflicts with hygienic validation requirements.
Hygienic heat exchangers are designed to:
Prevent bacterial growth
Eliminate dead zones
Allow full drainage
Withstand CIP and SIP cycles
Use FDA-approved materials
Industrial heat exchangers may prioritize cost and performance but do not necessarily meet sanitary regulations.
316L offers:
Better resistance to chlorides
Improved corrosion resistance under cleaning chemicals
Lower carbon content, reducing risk of weld sensitization
In pharmaceutical and dairy processes, 316L is generally the standard.
Common certifications include:
FDA (Food contact compliance)
3-A Sanitary Standard
EHEDG certification
ASME BPE (pharmaceutical standard)
GMP compliance
Certification requirements vary by region and industry.
Non-certified gaskets may:
Leach harmful substances
Degrade under cleaning chemicals
Absorb product and promote bacterial growth
FDA-certified elastomers ensure compliance with food-contact safety regulations.
A fusion-bonded heat exchanger is manufactured by laser welding or vacuum welding stainless steel plates without using copper or nickel filler metals.
This results in:
100% stainless steel wetted surfaces
No filler contamination risk
Higher hygienic reliability
Nickel-brazed units offer better corrosion resistance than copper-brazed units, but they are still generally not accepted in high-purity pharmaceutical processes due to inspection limitations and regulatory concerns.
Fully welded stainless steel designs are preferred.
Surface finish (Ra value) affects cleanability and bacterial adhesion.
Typical sanitary requirements:
Ra ≤ 0.8 μm (food industry)
Ra ≤ 0.6 μm or lower (pharmaceutical)
Smoother surfaces reduce microbial retention and improve CIP effectiveness.
Potential risks include:
Product contamination
Regulatory audit failure
Production shutdown
Legal liability
Brand damage
In regulated industries, material choice is directly linked to compliance risk.
Not necessarily.
Gasketed units allow inspection and mechanical cleaning
Fully welded units eliminate gasket aging risk
The choice depends on process pressure, temperature, and cleaning requirements.
Hygienic heat exchanger material selection must prioritize stainless steel construction, FDA-certified gaskets, and fusion-bonded design to ensure compliance with food and pharmaceutical regulations.